The first all-in-one HIV combo, mixing Viread (tenofovir), Emtriva (emtricitabine) and Sustiva (efavirenz) in a single once-a-day pill, filed for FDA approval in April and could hit pharmacies any day. It’s already delivered a new marketing term—single-tablet regimen, or STR: It stuffs an entire HIV combo into one tablet. Two companies, Gilead Sciences (maker of Viread and Emtriva) and Bristol-Myers Squibb (Sustiva’s manufacturer), teamed up to parent the triplet. To get it to other parts of the globe, Gilead will need to work with Merck, which sells efavirenz as Stocrin in some countries. All three drugs remain available separately; Viread and Emtriva also appear together as Truvada.
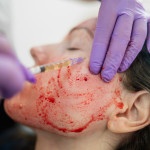
Bio-Alcamid, a filler to fix the facial wasting damage caused by some HIV meds, has been approved in Canada. Unlike Sculptra (the only HIV facial wasting remedy approved in the U.S.), Bio-Alcamid isn’t temporary and usually doesn’t need reapplication. The synthetic gel, injected under the skin with local anesthesia, has been available in Europe and Mexico for several years. Until it’s approved here (the maker predicts a mid-2007 U.S. clinical trial), folks can get treated up north with a doctor’s referral.
A patient-assistance program to help pay the cost (about $3,500 to $4,500, according to a company rep) is available to U.S. as well as Canadian residents; check www.faceforward.ca.
Sneak Previews
New treatments for HIV and its side effects





Comments
Comments