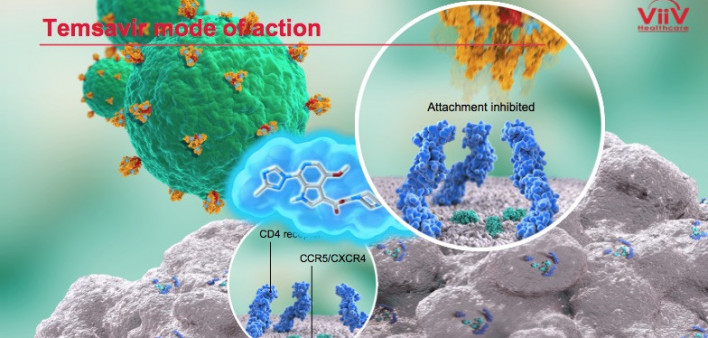
The addition of ViiV Healthcare’s investigational attachment inhibitor fostemsavir to an optimized antiretroviral (ARV) background regimen fully suppressed HIV in a majority of people with extensive prior treatment and highly drug-resistant virus 96 weeks into a recent study, the National AIDS Treatment Advocacy Project (NATAP) reports.
Max Lattaillade, DO, MPH, head of clinical development at ViiV, presented findings of the BRIGHTE Study at the 10th International AIDS Society Conference on HIV Science (IAS 2019) last week in Mexico City.
The study authors recruited people with HIV who had taken considerable number of ARVs and who were currently on a failing regimen. These individuals were put in the randomized cohort if they had one or more approved ARVs from one or two classes that were still fully active against their virus. Those whose virus was not susceptible to any approved ARVs were put in the nonrandomized cohort.
The 272 people in the randomized group either received 600 milligrams of fostemsavir twice daily or a placebo on a blinded basis, meaning they did not know whether they were receiving the active drug. All those in this group also stayed on their failing ARV regimen for the first eight days of the study. After that point, everyone in the randomized cohort received fostemsavir plus a background ARV regimen optimized to work as well as possible.
The 99 members of the nonrandomized group essentially skipped that first eight-day period and went right to receiving fostemsavir plus an optimized background regimen, which could include other investigational drugs.
At the outset of the study, the median age of the randomized and nonrandomized groups were 48 and 50 years old, respectively. They had median viral loads of 50,100 and 20,000, respectively, and median CD4 counts of 99 and 41, respectively.
Nearly all those in the nonrandomized cohort had virus that was not susceptible to any class of ARVs. Of those in the randomized group, 88% had exhausted nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), 81% had exhausted non-nucleoside reverse transcriptase inhibitors (NNRTIs), 74% had exhausted protease inhibitors, 29% had exhausted integrase inhibitors, 78% had exhausted CCR5 receptor antagonists and 85% had exhausted fusion inhibitors.
Fifty-two percent of those in the randomized group had just one ARV available that was fully active against their virus, and 42% had two such ARVs available. Six percent did not have any active ARVs they could take.
As previously reported, at 48 weeks into the study, 54% of the randomized cohort had a viral load below 40. The same proportion had a fully suppressed viral load at week 72, and 60% did so at week 96.
In the nonrandomized cohort, 38% had a fully suppressed viral load after 48 weeks and 37% did so after 96 weeks of treatment.
At week 96 of the study, the average CD4 count rose by 205 in the randomized group and 119 in nonrandomized cohorts.
Among those in the randomized and nonrandomized cohorts, a respective 21% and 22% experienced a drug-related adverse health event that was Grade 2 to 4 (moderate to severe), while a respective 34% and 48% experienced any serious adverse events and 5% and 12% experienced any adverse event that led them to drop out of the study. A respective 4% and 17% died. Most deaths were among people with a CD4 count below 50.
“BRIGHTE results support continued development of fostemsavir as an important treatment option for heavily treatment-experienced people living with multidrug-resistant HIV-1,” the researchers concluded.
To read the study abstract, click here.
To read the NATAP report, click here.





Comments
Comments