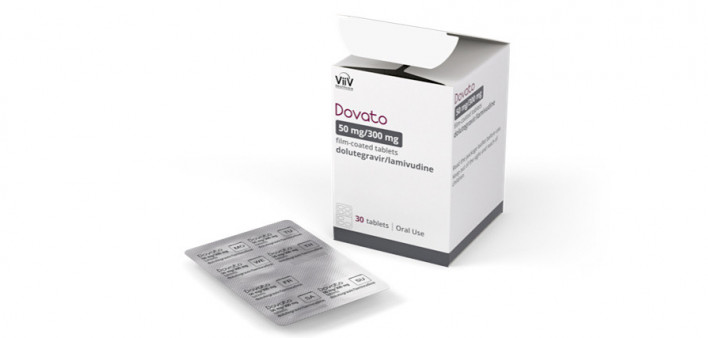
ViiV Healthcare, an HIV pharmaceutical company, has announced that the combination tablet Dovato (consisting of dolutegravir and lamivudine) is now available in a discreet, portable blister pack in the United States.
Dovato is a once-daily, single-tablet HIV medication that helps people living with the virus achieve an undetectable viral load. Viiv Healthcare, majority owned by GSK, created the blister pack based on insights from the HIV community, according to a ViiV news release.
“The Dovato blister pack is designed to help address some of the challenges we hear from the HIV community, which include stigma and convenience, and offers a discreet package [that] may fit more seamlessly into people’s daily routines,” said Lynn Baxter, head of North America at ViiV Healthcare.
“Everyone has different experiences and preferences when it comes to their HIV treatment, and at ViiV Healthcare, we are pleased to offer a variety of treatment and packaging options that help suit the needs of people living with HIV.”
The Food and Drug Administration (FDA) first approved Dovato as an all-in-one HIV treatment in 2019. The Dovato blister pack, which provides barrier protection for shelf-life requirements and a degree of tamper resistance, comes in a 30-count box containing five sheets of tablets about the size of a credit card. The blister pack received FDA approval in November 2023.
ViiV Healthcare aims to release the Dovato blister pack in some European markets in 2024 and will continue the sale of the medication in the current 30-count pill bottle.
Click here for a printable version of the POZ 2023 HIV Drug Chart.
To read more, click #Dovato. There, you’ll find headlines such as “Switching to Dolutegravir and Lamivudine Works Well Despite Resistance Mutations,” “Is Dolutegravir Alone Enough to Control HIV?” and “Treatment: Rapid Dovato.”






Comments
Comments